
Is NH3 Polar or Nonpolar? Techiescientist
Examples of such molecules include hydrogen sulfide, H 2 S (nonlinear), and ammonia, NH 3 (trigonal pyramidal). To summarize, to be polar, a molecule must: Contain at least one polar covalent bond. Have a molecular structure such that the sum of the vectors of each bond dipole moment does not cancel. Properties of Polar Molecules
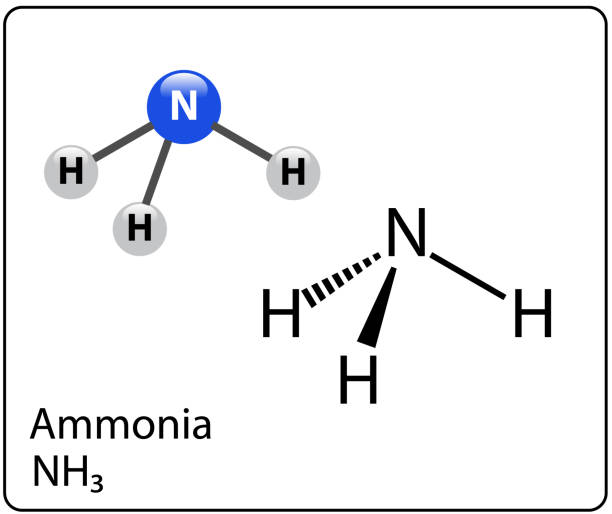
Is NH3 polar? How To Discuss
When you place a molecule with an electric dipole in an electric field, a force acts to turn the molecule so that the positive and negative ends line up with the field. The magnitude of the turning force is given by the formula. µ = q × d. where q is the amount of charge and d is the distance between the two charges. µ is the turning moment.
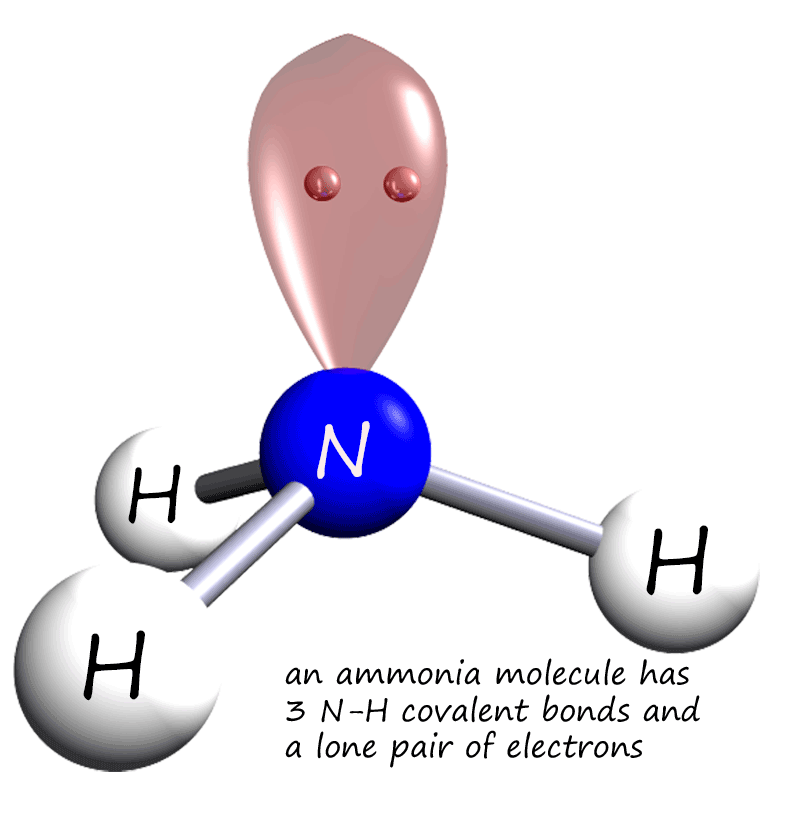
Covalent bonding
0:00 / 1:05 Is NH3 (ammonia) polar or nonpolar? Mentor Center 910 subscribers Subscribe Subscribed Share 13K views 3 years ago Chemistry videos Hey everyone, welcome to the Mentor Center! In.

Is NH3 Polar or Nonpolar?Is Ammonia a Polar or Nonpolar Molecule?
Polar molecules are molecules that have a permanent dipole moment. This permanent dipole moment is an indication of the molecule's polarity. The polarity of a molecule is determined by the distribution of electric charge within it. The electrons.

Is NH3 Polar or Nonpolar? Techiescientist
Ammonia has a molecular mass of 17.031 g/mol, maintains a density of 0.73 kg/cubic meter, has a boiling point of -28.01 degrees Fahrenheit, and a melting point of -107.9 degrees Fahrenheit. It is a stable binary hydride and is considered the simplest of the pnictogen hydrides.

Is NH3 Polar or Nonpolar?Is Ammonia a Polar or Nonpolar Molecule?
Ammonia: Propane, C 3 H 8: Bromine, Br 2: Solution. Ammonia is polar. When properly rotated, one can see that the nitrogen atom sits on a different plane than the hydrogen atoms . All of the arrows point in the direction of the nitrogen atom, giving it a net direction (the strength and direction of the arrows do not cancel).

Is NH3 Polar or Nonpolar?Is Ammonia a Polar or Nonpolar Molecule?
Due to the original pyramidal shape of the Ammonia molecule, it is polar in nature as its atoms share unequal charges. Check out the valuable article already written on the polarity of ammonia. Hybridization in Ammonia (NH3) Molecule. The bond between each nitrogen and hydrogen atom is covalent and made up of sigma (σ) bonds only and no pi (π.

Is NH3 (Ammonia) Polar or NonPolar? Lewis Structure YouTube
Ammonia is a pungent-smelling and colorless gas compound known by the chemical formula NH3 ie; having 3 molecules of hydrogen atoms and one nitrogen atom. At room temperature, it exists in the gaseous state and boils at -33 degrees Celsius. Many students wonder whether ammonia is polar or nonpolar.
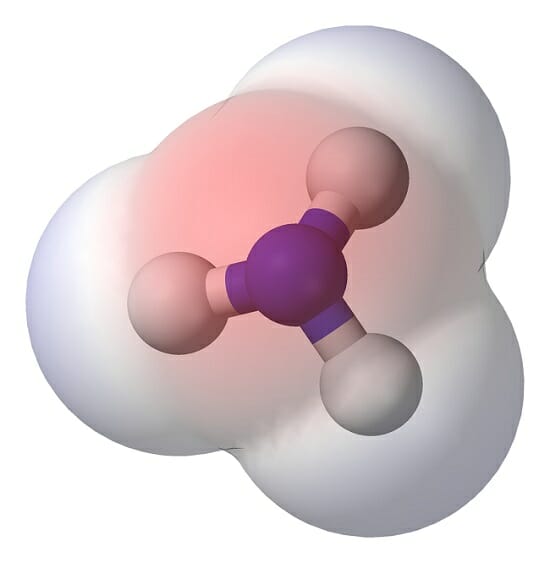
Polar Molecule Definition and Examples Biology Dictionary
To summarize, ammonia is a polar molecule because its electron geometry is trigonal pyramidal and the dipoles of N-H bonds do not cancel out. Remember, the net dipole of the molecule is the vector sum of all the dipoles and here it equals zero because the bonds are equivalent and pointing in opposite directions.
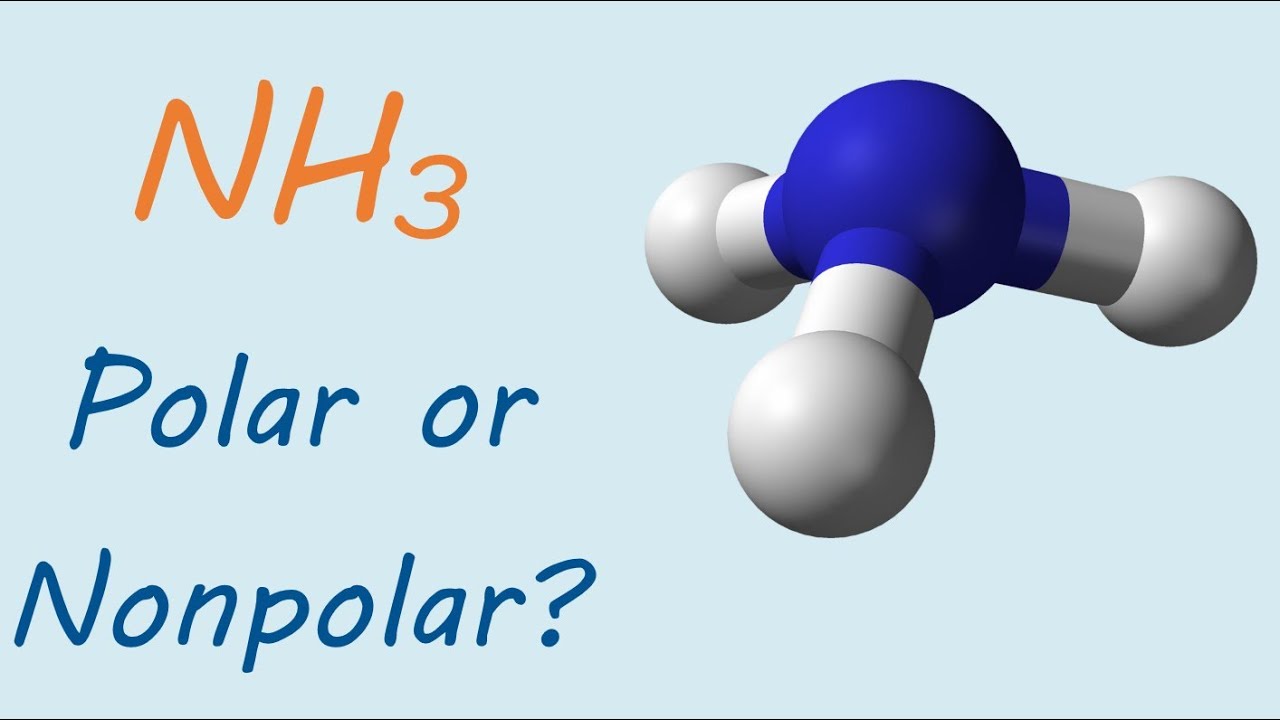
Is NH3 (ammonia) polar or nonpolar? YouTube
DO NOT FORGET TO SUBSCRIBE!LinkedIn: https://www.linkedin.com/in/kevan-j-e.Snapchat: https://www.snapchat.com/add/kravonoInstagram: https://www.instagram.c.

Ammonia Chemical Formula, NH3 Uses, Application, Valency
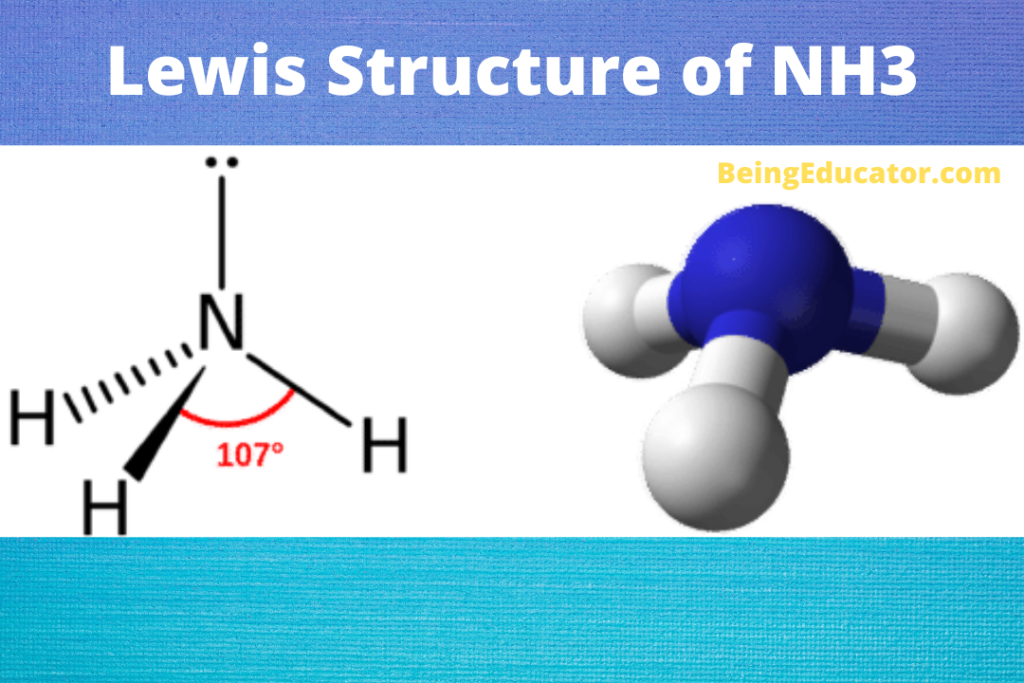
If you look at the Lewis structure for NH3 we can see that it is not a symmetrical molecule. However, to determine if NH3 is polar we need to look at the mo.
[Solved] explain why the ammonia molecule is polar while aluminum
Yes, Ammonia is a polar molecule. The polarity of nh3 is mostly depicted by the electron density difference between nitrogen and hydrogen atoms. The three N-H bonds each having separate dipoles makes ammonia a polar compound. The lewis structure of ammonia perfectly explains the polarity of nh3 as the three are a total of 8 valence electrons (5.
(Get Answer) Part A The ammonia molecule (NH3) is a polar molecule
ammonia is pyramidal 189 Electronegativity of N = 3.0, H = 2.1, so electrons in each bond are closer to the N than the H. Determine the resultant dipole. is the positive end. How about CH2Cl2 Determine Lewis Structure Use VSEPR to determine shape Determine the bond polarity The dipoles cancel out. No dipole moment.

Is NH3 Polar or NonPolar? (Ammonia) Yes Dirt
NH3 (or Ammonia) is a POLAR molecule because the Nitrogen (N) present in the molecule is more electronegative, which causes the partial positive (ẟ+) and partial negative (ẟ-) charge to appear on the molecule. These ẟ+ and ẟ- charges are responsible to make the entire NH3 molecule polar.
Is NH3 Polar or Non Polar (Simply Explained)
Solid NH 3 Two visible states of NH 3 Ammonia is a colourless gas with a characteristically pungent smell. It is lighter than air, its density being 0.589 times that of air.

Is NH3 Polar or Nonpolar? (Ammonia) YouTube
Ammonia or NH3 is a polar molecule as there is a large difference of electronegativities between Nitrogen and Hydrogen along with the asymmetric shape of the molecule. The uneven dispersion of electric charges in the molecule makes it a polar molecule. Priyanka To read, write and know something new every day is the only way I see my day!